The finding could pave the way for a new FDA-approved treatment for pre-treated mCRPC patients, up to 30% of patients of whom have tumors harboring these DDR mutations and may, therefore, benefit from PARP-inhibition.
This clinical trial is the first positive Phase 3 “precision medicine” trial to test targeted therapy among advanced prostate cancer patients whose cancer cells have specific gene mutations.
A “remarkable achievement”
PCF (Prostate Cancer Foundation)-funded investigator Maha Hussain from Northwestern University, Chicago, says achieving such a significant effect on disease progression and other clinically relevant effects such as pain progression and objective response rate among such heavily pre-treated patients with prostate cancer is remarkable:Prostate cancer has lagged behind all other common solid tumours in the use of molecularly targeted treatment so it is very exciting that now we can personalise an individual’s treatment based on specific genomic alterations in their cancer cells.”
Maha Hussain
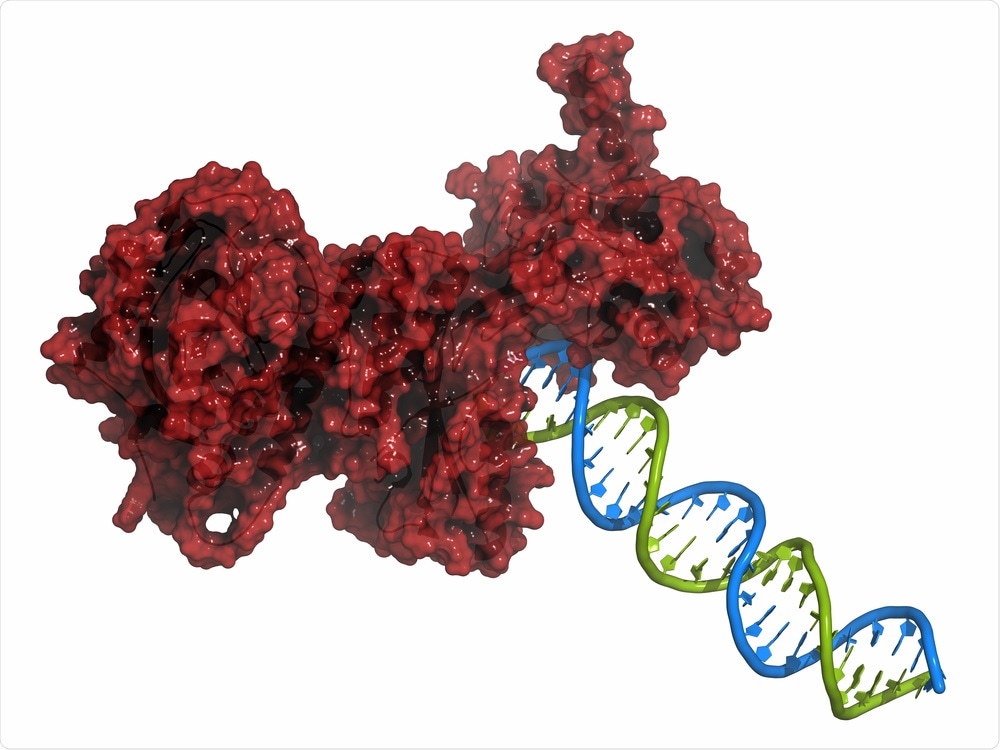
PARP-inhibitors are relatively new to oncology
Each year, 1.2 million men worldwide are diagnosed with prostate cancer, which causes death in more than 350,000 cases.Initial treatment approaches include surgery, radiotherapy, chemotherapy or hormonal treatment. However, treatment options are limited for patients with cancer that has spread to other parts of the body and become resistant to hormonal treatment (mCRPC).
PARP-inhibitors are a new class of drug that target the enzyme poly ADP ribose polymerase. They are FDA-approved for the treatment of breast and ovarian cancers that harbor mutations in the critical DDR genes that code for the tumor suppressor proteins BRCA1 and BRCA2, which fix damaged DNA.
Targeting DNA repair pathways in cancer cells
Cancer cells also rely on BRCA proteins to survive and if they have lost BRCA1, BRCA2 or one of many other DNA repair genes, they turn to the PARP enzyme to maintain sufficient integrity of their DNA.Therefore, cancer cells with mutations in BRCA1/2 are highly sensitive to PARP-inhibiting drugs, which increase the likelihood of tumor cell death.
The targeting of DNA repair pathways in cancer cells is an approach that has already been used to treat breast and ovarian cancer in patients with mutations in BRCA1 and BRCA2. Alterations in other DDR genes such as ATM can increase cancer cell susceptibility to PARP inhibitors.
The PROfound trial
In the PROfound trial, an international team of researchers compared the use of a PARP-inhibitor with the use of one of two new hormonal agents among two groups of men with mCRPC. Cohort A (245 men) had BRCA1, BRCA2, and ATM mutations and cohort B (142 men) had mutations in any one of 12 other less well-studied DDR genes.All patients had previously been heavily pre-treated. All had been treated with one of the two hormone treatments and just over two-thirds had been treated with chemotherapy.
The researchers report that in Cohort A, the median progression-free survival (PFS) was 7.39 months among patients taking the PARP-inhibitor compared with 3.55 months among patients taking a hormonal therapy.
In the overall population (Cohort A+B), the median PFS was 5.82 months with the PARP-inhibitor and 3.52 months with the hormone treatment. This represents a 66% reduction in risk of metastatic disease progression or death (whichever came first) in Cohort A, and a 51% reduction in the overall population.
At 12-months post-enrollment, 40% of Cohort A patients who had taken the PARP-inhibitor had no radiographic disease progression, compared with 11% of those who received hormonal therapy.
Hussain says the benefits of the PARP-inhibitor were observed in all subgroups of patients, irrespective of country, age, prior therapy and severity of the disease, including in those at a more advanced disease state that had spread to their liver or lungs.
“A landmark trial”
Eleni Efstathiou from the MD Anderson Cancer Center in Houston, USA, called the study a “landmark trial” because it is the first phase III trial to look specifically at tumors harboring a targetable molecular alteration.She says the 66% reduction in metastatic disease progression risk is impressive because it is “considerably higher than the 35-40% improvements with which we’ve been very satisfied in previous prostate cancer studies in this more advanced disease setting.”
Overall, these data show that, like breast and lung cancers, prostate cancer is not one, but many different diseases and we need to start identifying different groups of patients and treating them with targeted therapy.”
Eleni Efstathiou
The PCF says it is proud to have funded the foundational studies that provided the biologic and clinical rationale for this clinical trial and that the practice-changing findings will pave the way for a new precision medicine treatment option for many men with advanced prostate cancer.
The current study was funded by AstraZeneca and Merck Sharp & Dohme Corp.
Targeted Therapy Slows Progression of Advanced Prostate Cancer [ESMO 2019 Press Release]. September 30, 2019.






No comments
Post a Comment