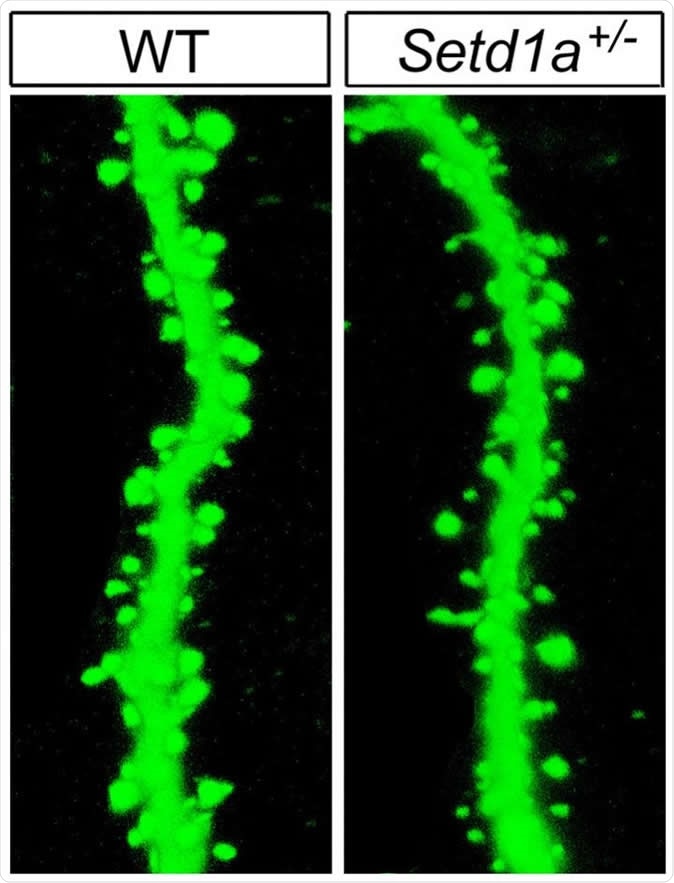
Mutant mice with impaired function of the SETD1A gene showed abnormalities in the neuronal machinery by which brain cells communicate. For example, there were fewer-than-normal spines (right), needed to relay signals, on branches of neurons - compared to those in wild type mice (left).
The researchers inactivated this gene, which they call “a master regulator”, in a mouse model, producing loss of working memory, just as in patients with schizophrenia. On the other hand, restoring the function of the gene brought the working memory back to normal function. In addition, reversal of this inactivation in adult mice also led to improvement of the affected neuronal circuits.
This shows that it may be possible to treat patients with schizophrenia due to SETD1A abnormality.
Researcher David Panchision says, “This schizophrenia risk gene codes for an enzyme that influences the expression of many other genes. In mice, a hobbled version of SETD1A disrupted gene expression in a network harboring other genomic suspects in schizophrenia. Remarkably, the resulting abnormalities were reversible.”
The SETD1A gene
The SETD1A gene is among the few that may be definitely associated with schizophrenia. It has been found to exist in several forms, some of which are rare while others are common. However, all of these increase the risk of schizophrenia, making SETD1A mutations one of the few genetic factors that are assuredly known to put the individual at risk for this psychosis. There are a host of other genetic changes that are more common in schizophrenia, but they contribute small individual effects to the risk. However, having just one abnormal SETD1A gene increases the schizophrenia risk markedly.SETD1A and experience-based learning
How is SETD1A involved in the brain’s cognitive processing? Scientists have found that this gene is vital in regulating genes involved in brain functioning via epigenetic markers. This means that experience ‘teaches’ the brain to change the pattern of expression of certain genes - equivalent to switching them on and off – by enzyme-mediated attachment of specific chemical groups at certain points to the DNA backbone. This is a process that occurs throughout the brain.Mutations in the SETD1A gene are rare, but most occur in patients with schizophrenia. This could help unravel the underlying mechanism that causes schizophrenia.
The study and its findings
The current experiment was set up in a mouse model, to explore the effects of a SETD1A mutation on the brain neurons, neural pathways, and host behavior. The mice used in the study had a mutation that resulted in only 50% of normal gene activity.These mice were then introduced to a task that involved threading a maze with a reward at the end. They failed to learn the task unlike normal mice - showing that their working memory was affected by the mutation. Working memory is the cognitive function that helps us to hold information in the memory, so that it can be used to guide behavior when required. When working memory fails, learning becomes impaired and tasks therefore become very difficult - a feature which is commonly observed in patients with schizophrenia.
Neuronal effects
At cellular level, dendritic growth and axonal branching are reduced by the mutation. Dendrites are the branch-like projections on neurons which receive incoming nerve impulses. Mice with the SETD1A mutation also had lesser numbers of dendritic spines (hairlike extensions from the dendrites). These changes delay the transmission of nerve impulses between adjacent cells because they impair the chemical-to-electrical conversion at the heart of nerve signal production.Gene transcription effects
Still more important was the discovery that the SETD1A mutation induced the disruption of many other linked genes that begin or increase the process of gene expression (‘promoters; and ‘enhancers’, respectively). This could help explain the changes in the neuronal structure and function. The researchers found that not just single genes but whole gene classes were expressed at a lower level while others were upregulated, in accordance to the level of networking with the gene. The protein expressed by the SETD1A gene is a lysine-methyltransferase gene, and binds to both enhancer and promoter sequences to modulate neuronal RNA synthesis. Many enhancers which bind SETD1A also bind the product of the MEF2 gene.One of these gene classes showed marked overlap with gene variants found in important pyramidal neurons of the cortex of the brain, in patients with schizophrenia. SETD1A binds to promoter sites to initiate transcription, while it binds to enhancers that have bound to MEF2 to produce the opposite effect. Thus the association of changes in two different sets of genes could therefore possibly cause a buildup of genetic effects on neuron structure and function, according to the researchers.
Reversal of effects
Finally, the scientists restored the expression of the SETD1A gene in adult mice to its normal level, and this in turn brought back normal working memory. The experiment also involved the use of an inhibitor which antagonized the action of the LSD1 gene (that counteracts the effects of SETD1A by removing the methyl group). This restored SETD1A actions, and brought about a complete correction of all the behavioral abnormalities as well as deficits in the neural circuits and communication.Many of these pathways are found in multiple species and have similar functions, which may show that they have similar roles in human schizophrenia.
Implications
The researchers are excited at the possibility that SETD1A reactivation or counteracting the brain changes consequent on SETD1A deficiency (such as by using LSD1 inhibitors, as in this experiment) could one day help to restore cognitive impairment in schizophrenia. Researcher Joseph Gogos explains, “Although SETD1A mutations exist in a small percentage of all schizophrenia patients, many people diagnosed with the disorder have issues similar to those caused by this mutation. Thus, therapies that are specific to SETD1A may indeed have wider implications for schizophrenia as a whole.”
Journal reference:
Jun Mukai, Enrico Cannavò, Gregg W. Crabtree, Ziyi Sun, Anastasia Diamantopoulou, Pratibha Thakur, Chia-Yuan Chang, Yifei Cai, Stavros Lomvardas, Atsushi Takata, Bin Xu, and Joseph A. Gogos. Recapitulation and reversal of schizophrenia-related phenotypes in Setd1a-deficient mice. Neuron. https://doi.org/10.1016/j.neuron.2019.09.014 . https://www.cell.com/neuron/fulltext/S0896-6273(19)30787-1






No comments
Post a Comment