Pivotal Trial of MRI-Guided Transurethral Ultrasound Ablation (TULSA) in Patients with Localized Prostate Cancer.
Most traditional treatment techniques rely on surgery or radiation therapy, but these may not always do the trick. In addition, they may result in serious adverse effects such as incontinence of urine, impotence and bowel issues.
Other less popular techniques such as radiofrequency or laser ablation, or cryotherapy, have not been widely adopted because of their need for advanced imaging guidance and temperature monitoring.
.jpg)
This diagram shows controlled and precise ablation using TULSA-PRO®.
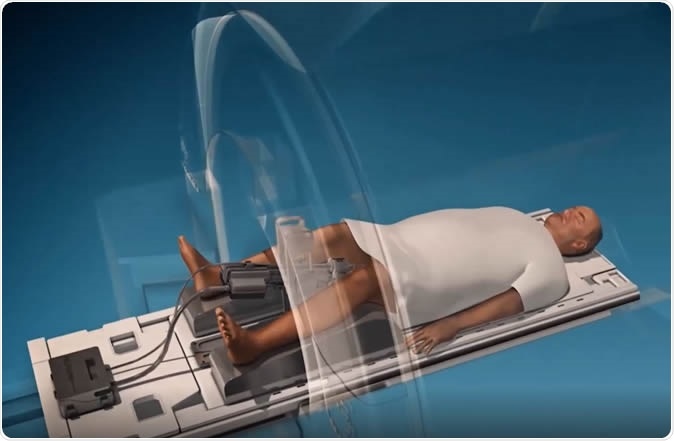
MRI-guided ultrasound
A newer method which could hold great potential for treatment is MRI-guided transurethral ablation, or TULSA. This method is minimally invasive and is based on the accurate placement of ultrasound waves at precisely calculated dosages, to prostate tissue that shows abnormalities, but without harming the surrounding healthy tissue.The use of high-intensity focused ultrasound waves to heat up cancer cells until they are destroyed is currently the least invasive method to physically remove cancerous tissue from this gland. The MRI images are fused to the ultrasound images, to guide the beam of ultrasound with the utmost precision. This treatment is limited at present to cancers that have not spread beyond the prostate, but can be used before or after other treatments, or after a failed radiation therapy.
The study
The researchers carried out an analysis on the 12-month post-treatment outcomes, from the procedure as performed in multiple centers, under the TULSA-PRO® ablation clinical trial (TACT). The data was obtained from 115 men of median age 65 years. All had localized prostate cancer confined to the gland, with low or intermediate risk. All were treated using TULSA to the whole gland, over an average of 51 minutes.The findings
The researchers say the results of treatment with MRI-guided TULSA were impressive. The prostate gland volume shrank by over 90%, while impotence resulted in very few patients, and incontinence in virtually none.
How TULSA operates
The basis of TULSA is a rod-shaped emitter that goes into the male urethra, carrying 10 elements that can cover the entire area of the prostate gland with ultrasound waves. Depending on the location of the tumor, one or more of these ultrasound generators are activated to emit ultrasound waves in order to heat up the targeted tissue to the point of ablation, or destruction.The automated ultrasound beam’s strength, direction, and shape are regulated by a software program, that uses an appropriate algorithm to adjust these parameters. Moreover, the use of an MRI scanner to house the whole procedure ensures that doctors can see what is happening in real-time and evaluate how much of the tissue is being destroyed, and where, and to what depth.
Advantages
Says researcher Steven S. Raman, “Unlike with other ultrasound systems on the market, you can monitor the ultrasound ablation process in real-time and get immediate MRI feedback of the thermal dose and efficacy. You can control with much more finesse where you're going to treat, preserving continence and sexual function. Second, you can do this for both diffuse and localized prostate cancer and benign diseases, including benign hyperplasia.”This is an immense advantage, compared to traditional ultrasound systems used to treat prostate cancer. Even better, the non-invasiveness of the procedure means that it can be done on an outpatient basis, and the recovery time is almost nil.
Following TULSA, other treatments can still be adopted if required, including the repeated application of TULSA itself as well as surgery or radiation therapy. TULSA can also serve as a method to treat localized cancers noninvasively after radiation failure.
In addition, the study shows that MRI could be used to monitor patients who have undergone TULSA. The participants in the current study had an MRI at one year after treatment. the results had a 93% to 96% negative predictive value for residual cancer. In other words, if the MRI failed to pick up cancer, the chances that there was indeed none were as high as 93% to 96%, making it an accurate method to exclude recurrent disease in patients.
Implications
TULSA has been approved for use in prostate cancer in Europe, and in the USA, the latter only recently under the US FDA 510(k) clause. More studies will be needed to validate the early results, but if so, it could advance the treatment of both benign prostatic hyperplasia and prostate cancer dramatically.
https://press.rsna.org/timssnet/media/pressreleases/14_pr_target.cfm?id=2129.






No comments
Post a Comment