
These are found at high levels in asthma, Quincke’s edema, and anaphylaxis, conditions that are troublesome or downright dangerous. The study, published in the journal Cells, is intended to help advance the understanding of how these reactions occur and how they can be combated to improve the quality of life of the affected individuals.
Immunoglobulin E
Immunoglobulin (Ig) molecules are unique creations produced by a type of immune cell called the B cell or later, its mature form called the plasma cell. These molecules are designed to detect antigens, or recognition molecules that are present on the surface of foreign particles or bacteria, and which help the immune system to eliminate or neutralize them.When such a neutralization reaction becomes overactive or continues beyond the time that it is useful, we call it an allergic reaction. IgE is a type of immunoglobulin that is almost always found to be implicated in the genesis of an allergic reaction as well as in the late phase.
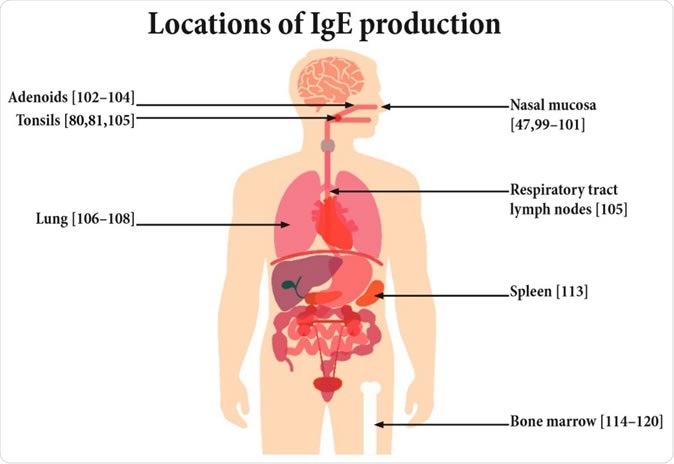
Potential sites of IgE production.
IgE is unlike other antibodies in that it is present at very low levels in serum (5x10-5 mg/mL) and has a very short duration of action. In fact, 50% of IgE molecules are destroyed within 2 days compared to 21 days with IgG1, another antibody class. The low concentration makes it hard to analyze these molecules.
Allergies could be hypothetically treated by removing IgE from the circulation, but the issue is the rapid return to normal or high levels. In other words, there is a continuous though low-level production of the molecule. This prompted the current attempt to localize and specify the cells from which IgE takes its origin.
Laboratory mice have often been used to create a model of allergy in order to understand how allergies develop and how they can be treated. While this is useful in exploring allergies, especially since mouse tissue can be obtained for experimental use with relative ease, while human tissue is hard to obtain, mice and humans are different in many ways that involve the induction of an allergic reaction and the mechanisms of allergy.
In addition, a laboratory mouse allergy model differs in significant ways to the multifaceted exposure to allergens that induce and modulate the immune response. This applies to the mechanisms by which IgE concentrations rise in allergies as well.
IgE producing immune cells are ‘memory’ cells that remember how allergens look for many years. As a result, the next time they are exposed to antigens, IgE levels shoot up very rapidly. This finding has led to the hypothesis that there are two specific mechanisms of IgE production: the setting up of a permanent IgE ‘plant’ that keeps churning out these molecules, which is perhaps composed of plasma cells; and an allergen-induced surge in IgE production.
Isolation of the cell of origin of IgE
The cells are treated with fluorescent antibodies, that will latch on to corresponding structures on the surface of B cells and IgE receptors to identify them by the resulting fluorescent glow. The glowing cells are then counted. However, this technique picks up so many falsely positive IgE-bearing cells that only about 0. 0019% of the isolated B cells are actually IgE-producing cells.
The researchers suggest the use of a fluorescent monoclonal IgE antibody that will differentiate IgE merely bound to the receptors from that which is actually bound to the membrane, that is, produced by the cell itself.
Prior research has shown that only about 0.2% of serum IgE is produced by plasma cells. The overwhelming majority comes from other cells located in various peripheral sites, from the mucous lining of the nose that makes initial contact with allergenic molecules, to the lungs and the tonsils. It is also produced in the spleen and bone marrow.
The cells at peripheral locations are, however, renewed from the cell populations circulating in the blood. Thus, the overlap between the blood-derived cells and the native tissue cells makes it a challenge to isolate the cells that are chiefly responsible for the production of IgE.
IgE-mediated T cell activation
IgE binds to an allergen to form a complex that binds to the CD23 molecule on the B cell surface, causing them to engulf the complex and subsequently process and present the allergen-derived peptide. This is recognized by specific T cells and is called IgE facilitated allergen presentation. This stimulates T cell activation and proliferation.Therapies directed against IgE
IgE levels can be lowered by newly synthesized drugs, which inhibit either the molecules or the cells that produce them. In the first case, the drugs bind to certain specific parts of the IgE domain that determines the attachment of the molecule to the immune cells that carry out the task of neutralizing, killing or breaking down the foreign antigen-carrying particles or cells. They thus restrict the amount of active IgE molecules that can connect with these effector cells, and in turn this reduces the amount of active inflammation that can occur as a result of effector cell degranulation.In the second case, the B cells that produce IgE are blocked by the binding of the drug to the IgE on the cell surface, tagging the cell for identification by effector T cells that destroy the IgE-producing cells.
The article concludes with the observation that there is a lot to learn about allergy mechanisms and IgE synthesis in order to design more effective therapies. Knowing the source of IgE production, the location of such cells, the mechanisms that cause an increase in IgE levels when the body is exposed to an allergen, and the speed of receptor stimulation in response to such changes, are crucial questions that still beg answers.
Journal reference:
Julia Eckl-Dorna, Sergio Villazala-Merino, Nicholas James Campion, Maria Byazrova, Alexander Filatov, Dmitry Kudlay, Antonina Karsonova, Ksenja Riabova, Musa Khaitov, Alexander Karaulov, Verena Niederberger-Leppi and Rudolf Valenta, Tracing IgE-Producing Cells in Allergic Patients, Cells 2019, 8(9), 994; https://doi.org/10.3390/cells8090994, https://www.mdpi.com/2073-4409/8/9/994/htm.






No comments
Post a Comment