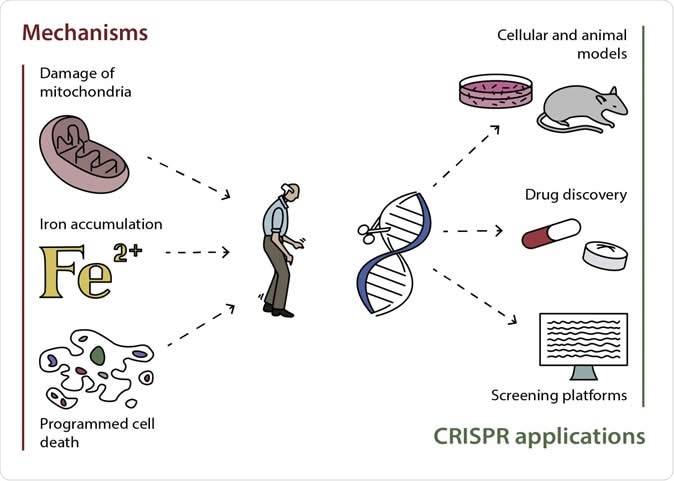
Possible mechanisms of development of Parkinson's disease and the most promising ways of applying CRISPR/Cas9 for its studying and treating
People with Parkinson’s disease experience loss of dopamine-producing brain cells, leading to tremors, rigidity, bradykinesia, and postural instability, the primary symptoms of the disease.
The loss of dopamine-producing neurons is a pathologic hallmark of Parkinson’s disease. However, the exact mechanisms remain unclear. A team of researchers at the Sechenov University and University of Pittsburgh unveiled the most promising strategies in applying genetic engineering in a study and treatment of Parkinson’s disease.
The noble method can help study the role of cellular processes in the disease progression, develop new treatment methods and drugs, and estimate their effectiveness using animal disease models.
Parkinson’s disease (PD) is a neurodegenerative disorder that affects one’s motor skills, including balance and coordination. Across the globe, about 6.5 million people have Parkinson’s disease in 2016, compared to 2.5 million in 1990.
In Australia, the prevalence of people with PD was higher than many cancers in 2014, and an estimated one in every 308 people lives with Parkinson’s.
Brain mechanisms trigger disease progression
The neurodegenerative disorder has many motor and cognitive impairments and is usually seen among older adults, who are more than 55 years old. The disease progresses slowly and gradually, as it worsens over time. In severe cases, the patient may experience difficulty in controlling their motor skills and movements, talking, and independent actions, such as taking care of themselves.A noble treatment is important to help improve the quality of life of those inflicted with the disease. Published in the journal Free Radical Biology and Medicine, the study highlights how mechanisms in the brain trigger the development and progression of PD.
hey found many mechanisms that seem to trigger and hasten the development of Parkinson’s disease. They discovered that 10 percent of all cases of PD are genetically predetermined. In most cases, the conditions stem from both genetic and environmental risk factors.
While the aberrant redox metabolism is linked to iron dysregulation and accumulation of impaired mitochondria and is considered as one of the major drivers for neurodegeneration and death of dopaminergic cells, the specific mechanisms and anomalies are still unclear.
The researchers focused on probable causes of the disease, which are associated with the oxidation-reduction (redox) reactions in cells, and the mechanisms of cell death, dubbed as apoptosis and ferroptosis.
CRISPR, a genetic engineering technology, is a promising method to find new and effective drugs and treatments for neurodegenerative disease. There are many ways CRISPR can help address Parkinson’s disease.
Mitochondria and Parkinson’s disease pathology
Since mitochondria damage can lead to progressive error in the cell, it’s important that aged and damaged mitochondria should be eliminated immediately, in a turnover called mitophagy. When there is excess or inefficient mitophagy, it can lead to neurodegeneration.
People with a mutation in the genes for PINK1 and Parkin, which are proteins important for mitochondrial functioning, had muscle degeneration, mitochondrial dysfunction, and loss of dopaminergic brain cells, which is typical to Parkinson’s disease.
In this scenario, CRISPR can help search for unknown genes and their protein products, which are drivers for disease development and progression. These proteins can be targets for the discovery of noble drugs and therapies, in the aims of treating people with PD.
3d illustration of CRISPR-Cas9 technology
Iron homeostasis in cell functioning
Homeostasis is the internal balance of body processes. The second group of cellular processes is associated with iron homeostasis or balance. Iron and ROS are important for the proper functioning of the cells, and if there is any alteration, it could lead to disruptions in vital cellular processes.If there is an imbalance in iron, it can accumulate in the brain tissues of older adults. Usually, they can deposit in areas of the brain that controls motor and cognitive functions. In previous studies, high level of iron in the substantia nigra is accompanied by dopaminergic brain cell death.
CRISPR technology can help develop drugs that can normalize iron balance in the affected areas of the body, in this case, the brain.
Apoptosis and ferroptosis
The last group of processes is cell death programs, called apoptosis and ferroptosis. The cells’ proteins and DNA are broken down with enzymes during apoptosis until it disintegrates. After which, ferroptosis occurs, wherein the iron-dependent oxidation of lipid classes. Oxidation byproducts accumulate in the cell, which leads to poisoning. These cell death programs may contribute to the development of neurodegenerative diseases, since they speed up the death of cells, including the dopaminergic neurons. CRISPR can help in this case by providing detailed studies of cell death pathways, leading to a better understanding of their roles in the development of Parkinson’s disease and other brain diseases.“This is one important step of many toward bringing the promise of this new technology to patients with serious diseases like neurodegenerative disorders. It has already been used in human trials (in China, Germany, the USA) to treat patients with cancer at a late stage and beta-thalassemia,” Margarita Artyukhova, study author, said.
“Such studies allow us to see the vast potential of genome editing as a therapeutic strategy. It's hard not to be thrilled and excited when you understand that progress of genome editing technologies can completely change our understanding of the treatment of Parkinson's disease and other neurodegenerative disorders," she added.
Journal reference:
Artyukhova, M., Tyurina, Y., Chu, C., Zharikova, M., Bayir, H., Kagan, V., and Timashev, P. (2019). Interrogating Parkinson's disease associated redox targets: Potential application of CRISPR editing. Free Radical Biology and Medicine. https://www.sciencedirect.com/science/article/pii/S0891584919302515?via%3Dihub






No comments
Post a Comment